Managing Osteoarthritis and Pain The Role of COX-2 Inhibitors U.S. Pharmacist Continuing Education ACPE Program No. 430-000-99-009-H01 This program provides 2.0 hours of credit (0.2 CEU). Lesson Expires: September 30, 2001 This CE article is supported by an unrestricted educational grant from Merck & Co.
COX-2 specific inhibitors sideline the
typical GI adverse effects seen with other NSAIDs. Osteoarthritis (OA), also known as degenerative joint disease, is the most
prevalent form of arthritis and the second most common cause of long-term disability in
the United States.1-3 Nearly everyone has
osteoarthritis-related radiographic changes in the knees or other joints by age 75,
although most individuals have no symptoms.3
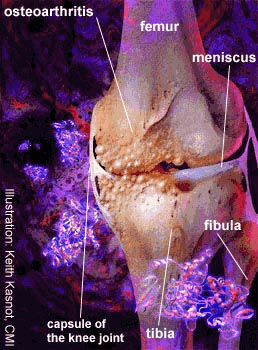
Osteoarthritis is characterized by focal loss of cartilage as well as hypertrophic bone
spurs. The name derives from the overgrowth of bone at the margins and subchondral areas
of the joint.4 In the past osteoarthritis was regarded as
a “wear and tear” form of arthritis, in which weight-bearing joints simply wore out or
“degenerated” with advancing age. However, the disease is now viewed as an active
process in which specific biochemical changes occur in joint fluid, cartilage and
subchondral bone.3 Some of the biochemical processes are
thought to be reparative responses to normal joint insult, leading to the hope that these
changes can be manipulated toward improved outcomes. It is uncertain what initiates degradation of cartilage, but it may be mechanical stress that leads to microfractures of cartilage, increased stress on surrounding tissue and induction of altered chondrocyte metabolism to favor proteolytic enzymes such as matrix metalloproteinases.3 Once initiated, the process becomes self-perpetuating. The increased stress on subchondral bone leads to formation of osteophytes, or spurs of new bone. These bone spurs result in the hard, bony enlargement that is a clinical characteristic of osteoarthritis. The main symptom that patients complain of is pain, especially with use of the joint.5 Other symptoms include joint stiffness, limitation of movement, variable degrees of local inflammation and loss of function (TABLE 1). On examination, joints may have some localized tenderness and firm swellings at the joint margins (due to the bony hypertrophy). Unlike rheumatoid arthritis, osteoarthritis may affect joints asymmetrically. There are no systemic symptoms outside the joint. In most patients with mild osteoarthritis, the disease does not progress to severe joint damage.4
There is no cure for osteoarthritis. Rather than attribute all joint
symptoms in the elderly to osteoarthritis, the clinician should rule out other causes of
pain.1,2,4 When treating for osteoarthritis, goals have
been suggested as three-fold.6 The first is management of
pain and other symptoms. The second is the need to maintain or improve function and the
third, so far unachievable, goal is to modify the long-term biochemical processes of
osteoarthritis. Since the main indication for drug therapy in OA is pain, this article
will focus on the management of pain. A major component in acute pain is the detection of tissue damage by activation of pain receptors in thin myelinated A delta or nonmyelinated C fibers, which then transmit a message to the brain.8 However, the perception of pain stimuli is affected by inflammatory and neural changes in the immediate environment.7 The threshold for activation of these nociceptors can be regulated by several inflammatory mediators, including prostaglandins, bradykinin, serotonin and histamine.8 Several peptides, such as substance P, are contained within the primary afferent pain fibers and can be altered by sustained stimuli or damage to the nerve. (Pain can also be caused by muscle contraction, as in the painful uterine cramping of dysmenorrhea where excessive prostaglandin F2 is released and stimulates contraction.) The basis for analgesic action of nonsteroidal anti-inflammatory drugs (NSAIDs) is their ability to prevent production of prostaglandins, particularly prostaglandin E2.8 Local injury or inflammation increases cyclooxygenase-2 (COX-2) activity, which leads to prostaglandin E2 synthesis and hyperalgesia.9 Nonsteroidal anti-inflammatory drugs were once thought to act only at local sites of pain reception. However, prostaglandins also facilitate transmission of pain in the spinal cord, and painful stimuli have been shown to increase COX-2 expression within the central nervous system.9 Induction of COX-2 has been established at both local and central sites of pain reception and transmission. In animal studies COX-2 inhibitors have been shown to act both at local sites of nociception and centrally when administered by intrathecal injection.9 Acute pain usually decreases and ceases before the body has completely healed because it is so dependent on these inflammatory sensitizers. In contrast, pain becomes chronic when it persists beyond the expected healing time, for longer than three months. Chronic pain is often triggered by injury or disease, but is frequently perpetuated by other factors. Because chronic pain is unrelenting, affective (i.e., psychological, social, or emotional) factors contribute to the intensity of pain. Community-based studies have shown that joint pain and disability in elderly people with osteoarthritis depend as much on factors such as isolation and depression as on severity of joint damage.4 Pain severity correlates poorly with radiographic features of OA. In chronic pain medical treatment is only one component of treatment, while nonpharmacologic treatments such as cognitive and behavioral therapy are also very important. Pain is the main symptom that OA patients complain of. Cartilage is vascular and has no nervous innervation, so pain is unlikely due to cartilage destruction.6 Pain in OA is probably due to irritation of surrounding structures outside the joint.6,10 Managing OA Pain Nonpharmacologic Treatment: Educating patients is important, for an informed patient is more likely to adhere to a treatment regimen. Modifying occupational and recreational activities, reducing weight and strengthening muscles may reduce stress on joints. Proper footwear and assistive devices, such as canes, may also reduce the mechanical forces across the joint.11 (See TABLE 2.) Some studies have shown that programs to strengthen specific muscle groups can modestly reduce pain and disability.11 A multicenter study that lasted 18 months showed that either aerobic or resistive exercise was better than education alone when combined with standard pharmacological therapy in patients with osteoarthritis of the knee.12
In advanced osteoarthritis of the knee or hip, surgical interventions, especially total joint replacement, offer excellent relief of pain and functional improvement.1,2 Topical Analgesics: In patients who do not wish to take oral drugs, or who want adjunctive relief, topical analgesics such as methylsalicylate or capsaicin cream are appropriate.2 Capsaicin, available as a topical cream in various concentrations, is a derivative of red chili peppers that depletes peripheral sensory nerves of the neurotransmitter called substance P. Studies in osteoarthritis have shown that capsaicin 0.075% can decrease pain and tenderness by about 40% when applied to specific joints four times daily for a month.10 A high-strength (0.25%) formulation of capsaicin is available that can be applied twice a day and provides faster and stronger pain relief.13 Drawbacks of capsaicin are the need for frequent application and a local burning sensation that will occur for the first few days. Acetaminophen: Based on cost, efficacy and safety, acetaminophen as needed up to 4 g/day should be the first-line pharmacologic therapy for osteoarthritis.1,2,6 Adverse effects are minimal, although patients who consume alcohol regularly need to be monitored for development of liver toxicity. Long-term use of acetaminophen has been associated with renal failure, but this also can occur with nonsteroidal anti-inflammatory drugs (NSAIDs). Acetaminophen is better than placebo in OA.14 Efficacy of acetaminophen has been compared and found similar to NSAIDs for osteoarthritis of the knee in several double-blind studies.1,2,15 For example, Bradley et al. compared acetaminophen 4 g/day, low-dose ibuprofen (1,200 mg/day) or high-dose ibuprofen (2,400 mg/day) in 184 patients with osteoarthritis of the knee and found all treatments to be equivalent in efficacy. Opioid Analgesics: Guidelines suggest that drugs such as propoxyphene, codeine or oxycodone can be helpful for acute exacerbations of pain but should be avoided for long-term use.1 However, NSAIDs do not always provide adequate analgesia or are poorly tolerated. Therefore, some authors suggest that opioids be considered earlier in the treatment plan, particularly for patients at high risk of NSAID-induced side effects.16 Oxycodone—alone or with acetaminophen—has been demonstrated to be better than placebo in treating osteoarthritis pain.16 Opioids work primarily in the central nervous system to reduce the perception of pain. Occasional doses may induce sleepiness, dizziness or constipation but otherwise are safe for short-term use. Chronic narcotic use is controversial due to the potential for inducing physical and psychological dependence. A decision to prescribe these drugs regularly should be based on the severity of pain, lack of effect of other drugs, and a clear understanding by the patient of permissible maximum daily doses. With careful supervision by a single physician and pharmacist, use of these drugs sometimes may be a reasonable option for pain not relieved by any other approach. Hyaluronic Acid: Hyaluronic acid is a substance in joint fluid that gives it viscosity. In osteoarthritis, the molecular weight and concentration of hyaluronic acid are diminished. Two new medical products have been approved for viscosupplementation in osteoarthritis. Both are believed to work as lubricants by substituting for hyaluronic acid in the joint. Because of this mechanism of action, they were each approved by FDA for osteoarthritis of the knee as medical devices rather than as drugs. The two supplements are similar, but not the same. Hyalgan is sodium hyaluronate, and Synvisc is hylan G-F 20, a longer-acting polymer of hyaluronate. Hyalgan is administered in a series of five joint injections, Synvisc in three. Even though the supplements are cleared from the joint within days, pain-relieving effects of either agent last for approximately six months. The effectiveness of these supplements is no better than that associated with NSAIDs, but they allow patients to avoid the adverse effects of NSAIDs.17 The supplements seem to be most useful in mild to moderate osteoarthritis; they are not as useful in advanced disease and do not prevent the need for joint replacement surgery. Systemic adverse reactions with hyaluronic acid injections are rare, and local pain and swelling from the injections are usually mild and transient. Glucosamine and Chondroitin: These dietary supplements have received much public attention. Glucosamine is a precursor to glycosaminoglycans in cartilage, and chondroitin is a component of glycosaminoglycans. A number of small, double-blind, controlled studies have shown that glucosamine can reduce OA-related pain. It may take two weeks to two months before significant reduction in pain occurs, but by that time pain reduction is comparable to that from NSAIDs.18,19 Very few studies of chondroitin alone exist, and the efficacy of this supplement is less well established. The supplements appear to have few side effects at usual doses. The most common complaint is nausea or mild gas. Typical doses are 500 mg TID of glucosamine and 1,200 mg of chondroitin daily. Glucocorticoids: Systemic glucocorticoids are not indicated for osteoarthritis, but intra-articular injections in the knee may be beneficial for patients who have effusion and local signs of inflammation.2 Pain relief and reduction of swelling may last up to four weeks.11,14 The interval between injections should be at least three to four months, due to concerns of adverse effects on cartilage metabolism.2,11 Disease-Modifying Drugs: Modifying the osteoarthritis disease process by stimulating cartilage repair or inhibiting breakdown would hold great promise. Proteolytic breakdown of cartilage is mediated by collagenase and matrix metalloproteinases (MMPs). Tetracyclines have been shown to inhibit MMPs and may have a chondroprotective effect. A multicenter trial examining the efficacy of tetracycline is underway.11 Additional metalloproteinase inhibitors—collagenase inhibitors and growth factors—are being investigated as disease-modifying drugs in osteoarthritis. Nonsteroidal Anti-inflammatory Drugs (NSAIDs) According to the American College of Rheumatology guidelines, NSAIDs are indicated for osteoarthritis patients who do not respond to acetaminophen and topical analgesics.2 NSAIDs were traditionally the drugs of choice in osteoarthritis,1 and most primary care physicians still choose NSAIDs first.20 However, the drugs’ ability to provide symptomatic relief is not necessarily better than that obtained with acetaminophen, probably because inflammation plays little role in osteoarthritis. There is also some concern about deleterious effects on cartilage metabolism. However, the main reason that NSAIDs are not employed as first-line therapy is their toxicity. There are many NSAIDs to choose from, but no evidence indicates that one is more effective than another in OA.14 Toxicities are comparable too, but nonacetylated NSAIDs (e.g., salsalate, choline magnesium trisalicylate) have less renal toxicity and antiplatelet effects. Since intensity of OA pain varies from day to day—and even within a day—using short-acting NSAIDs as needed is a reasonable approach.1 Low doses of NSAIDs should be used before higher ones are considered.1 Mechanism of Action: Nonsteroidal anti-inflammatory drugs inhibit the enzyme cyclooxygenase (COX), which is the first enzyme in the pathway that converts arachidonic acid into various prostaglandins. Prostaglandins are locally synthesized chemicals that have a role in inflammation as well as numerous other body processes. The nonspecific inhibition of prostaglandin synthesis throughout the body causes the wide range of adverse effects associated with NSAIDs. of NSAIDs The greatest problem with NSAIDs is that they may cause gastrointestinal bleeding and ulcers. Bleeding from NSAID-induced ulcers often occurs without warning and may be life-threatening. It is estimated that NSAIDs are responsible for 107,000 hospitalizations annually for GI complications and 16,500 NSAID-related deaths per year.32 Patients at higher risk of GI ulcers are the elderly, those with a previous history of ulcers, those taking high NSAID doses or concurrent glucocorticoids, and those with severe disability from arthritis or heart disease.1,24,25 One method of protecting the stomach is to use a concurrent drug that minimizes damage. Misoprostol (Cytotec; 200 mcg 4 times per day), an orally active prostaglandin analog, has been shown to significantly reduce incidence of both gastric and duodenal ulcers and to cut the risk of clinically serious GI bleeding by 38%.26 However, misoprostol frequently causes diarrhea or abdominal pain and should be reserved for patients at high risk of GI bleeding. Usual doses of histamine-2 receptor antagonists, such as cimetidine and ranitidine, prevent the development only of duodenal ulcers, not gastric ulcers. However, omeprazole 20 mg/day as well as misoprostol can prevent both ulcer types.25 NSAIDs may affect kidney function because the kidneys synthesize prostaglandins to help maintain blood flow in situations in which perfusion is reduced. Both COX-1 and COX-2 are found in the kidney.9 Problems are more common in patients who already have some intrinsic renal dysfunction or who have reduced renal blood flow (e.g., patients with congestive heart failure and the elderly). Some patients will simply develop some retention of sodium and water, resulting in weight gain or mild leg edema. Some develop worsening of hypertension. Occasionally, NSAIDs may cause acute renal failure by reducing the glomerular filtration rate (GFR). NSAIDs also interfere with platelet function. Inhibition of platelet aggregation may increase the risk of bleeding problems, including GI bleeding. In vitro studies show that NSAIDs can promote or inhibit cartilage breakdown by modifying proteoglycan and collagen synthesis, altering cytokine-induced cartilage resorption or by altering the release of matrix metalloproteinases.27 Indomethacin has been associated with accelerated joint destruction of the hip, and probably should not be used in osteoarthritis.1,27 Otherwise, no convincing studies have shown that NSAIDs have a beneficial or harmful effect in human osteoarthritis.1,27 As in other tissues, COX-2 can be induced by cytokines in chondrocytes, while COX-1 is constitutively expressed.28 Therefore, selective inhibition of COX synthesis may offer the hope of creating drugs with beneficial effects on cartilage. COX-2 Specific Inhibitors A recent survey of primary care physicians found that two-thirds would start therapy for osteoarthritis with an NSAID instead of acetaminophen.20 Furthermore, in patients with dyspepsia, most of these physicians would change NSAIDs or add an “antidyspeptic” drug.20 Clearly there is a role for safer NSAIDs in treating OA. Two new COX-2 specific drugs, celecoxib and rofecoxib, are now available for use. COX-2 specific drugs have sometimes been referred to as “super aspirins,” but there is no evidence that they are superior in effectiveness to current NSAIDs—only that they are safer. Mechanism of Specificity: When it was found that COX-1 and COX-2 enzymes were somewhat different in structure, scientists exploited the differences to design drugs with binding specificity. None of the previously marketed NSAIDs have enough COX selectivity to be clinically meaningful.29 COX-2 specific drugs all have rigid side chains that bind to a pocket found only in the COX-2 enzyme. COX-1 is necessary for platelet aggregation and GI protection; thus, a drug selective for COX-2 should be free of gastrointestinal and bleeding adverse effects. However, long-term safety and efficacy of these drugs is still to be established, as we do not understand all the possible physiological roles for either COX enzyme.
Since COX-2 is constitutively present in some organs, including the kidney and brain, and can be induced in other tissues, COX-2 specific inhibitors may not be free of side effects. In particular, the safety of these drugs in patients with renal impairment remains to be established. COX-2 is induced in the kidney in response to sodium depletion.9 COX-2 is also induced under stress in the brain, in the ovary during ovulation and implantation, and in colon adenoma and carcinoma cells. The distribution of COX-2 suggests that COX-2 specific inhibitors may have unique therapeutic niches in the future, such as in preventing colorectal cancer or Alzheimer’s disease.9 COX-2 is inducible in H. pylori-related gastritis and other gastric
injury.9 Thus, even if COX-2 specific inhibitors cause
fewer ulcers themselves, they may still inhibit healing of existing ulcers. Furthermore,
COX-2 specificity will not necessarily prevent GI symptoms such as nausea and abdominal
pain because these symptoms correlate poorly with markers of GI bleeding or ulceration.21 Use with Low-dose Aspirin: Many older patients need to take low-dose aspirin for cardiovascular protection. Low-dose aspirin may be used concurrently with COX-2 specific inhibitors because COX-2 inhibitors have no antiplatelet effect. However, any use of aspirin with celecoxib or rofecoxib could potentially increase the risk of GI bleeding.30,31 The role of COX-2 specific inhibitors will be primarily for patients who are at high risk for NSAID-induced ulcers. Their use would seem preferable to having to counter the side effects of NSAIDs with another drug, such as misoprostol or a proton-pump inhibitor. Since patients with osteoarthritis are generally elderly, and age is a risk factor for gastrointestinal ulcers, COX-2 specific inhibitors should be appropriate for many patients. At this time, the role of COX-2 specific inhibitors for patients with renal dysfunction is unknown. Celecoxib: Celecoxib (Celebrex) was approved in December 1998 for treatment of osteoarthritis and rheumatoid arthritis. The dose in OA is 200 mg once daily or 100 mg BID. The dose in RA is 100 to 200 mg twice a day. Celecoxib is moderately long acting, with a half-life of 11 hours. An osteoarthritis study involving 401 patients randomized subjects to receive placebo or celecoxib 25 mg BID, 100 mg BID or 400 mg BID for four weeks. The results showed that 100 mg and 400 mg BID doses were equivalent in efficacy. Each was superior to placebo and the 25 mg BID dose.32 Another 12-week, randomized, double-blind study was conducted in 1,004 patients suffering a flare of osteoarthritis of the knee. A dose of 50 mg BID of celecoxib was better than placebo but not as good as naproxen 500 mg BID. However, doses of 100 mg BID or 200 mg BID of celecoxib were equivalent to each other and to naproxen in efficacy.22 Celecoxib product labeling reports that 4,200 patients with OA of the knee or hip were studied in clinical trials at doses of 200 mg once a day and 100 to 200 mg BID. All doses were equally effective and reduced pain in 24–48 hours after starting therapy.30 Celecoxib has also been tested in a dental pain model.32
In a study of 200 patients who had impacted molar teeth removed, single doses of 100 mg or
400 mg of celecoxib were superior to placebo and at least as good as aspirin 650 mg in
relieving pain. Celecoxib has no effect on platelet aggregation. Its GI safety was tested in a rheumatoid arthritis trial that compared celecoxib 200 mg BID to diclofenac 75 mg BID for up to 24 weeks in 655 patients. Efficacy of the two drugs was similar. However, ulcers occurred in only 4% of celecoxib patients compared to 15% of diclofenac patients. Overall, GI complaints occurred in 36% of the celecoxib group versus 48% of the diclofenac group.33 The most common adverse effects of celecoxib are abdominal pain, diarrhea and dyspepsia. Occasional reports of increased prothrombin time have been described in patients taking warfarin after celecoxib was added.30 Since celecoxib was marketed, several deaths and cases of GI hemorrhage have been reported to FDA. In view of the nearly three million prescriptions written for celecoxib, it is possible that these adverse events were simply coincidental.34 However, these cases should serve as a reminder that rare cases of GI hemorrhage may occur even with COX-2 specific inhibitors. Rofecoxib: Rofecoxib (Vioxx) was approved by the FDA in May for osteoarthritis, acute pain in adults and menstrual pain. It is highly COX-2 selective, even at doses well above therapeutic.23 The recommended dose for osteoarthritis is 12.5 mg once a day, increasing to 25 mg/day if necessary. The recommended dose in acute pain is 50 mg once daily. Its half-life is about 17 hours. Use of rofecoxib for more than five days in the management of pain has not been studied.31 In a double-blind study of 104 subjects, rofecoxib 50 mg demonstrated single-dose analgesia equivalent to ibuprofen 400 mg after dental surgery. Mean onset of pain relief was less than 45 minutes.23 In unpublished studies of moderate to severe post-orthopedic surgical pain and primary dysmenorrhea, rofecoxib has been studied for durations up to 5 days.31 The analgesic activity of 50 mg/day was similar to naproxen sodium 550 mg or ibuprofen 400 mg. Rofecoxib in the Very Elderly: Unpublished data available from the manufacturer include a randomized, double-blind study of rofecoxib in 341 patients over 80 years of age (range 80–95 years) with knee or hip osteoarthritis. This is an important population, which is underrepresented in clinical trials. In terms of efficacy, rofecoxib at either 12.5 mg or 25 mg daily was more effective than placebo and equal to nabumetone 1,500 mg once daily. Rofecoxib was generally safe and well tolerated, even though most patients had comorbidity (75% had cardiovascular disease). Rofecoxib has been compared to older NSAIDs in osteoarthritis of the knee and hip at a dose of 12.5 mg or 25 mg once daily. Its efficacy and tolerability at both doses were similar to those of diclofenac 50 mg TID in a 6-month randomized, double-blind study of 784 patients.35 Another double-blind study, in 736 patients, that lasted 6 weeks found both doses of rofecoxib superior to placebo and comparable to ibuprofen 800 mg TID.36 In two open-label endoscopic studies of 6 months’ duration, rofecoxib 25 mg/day or 50 mg/day caused fewer gastroduodenal ulcers than did ibuprofen 2,400 mg/day, and no more ulcers than did placebo.31 The most frequent adverse events with rofecoxib have been headache, nausea, diarrhea and upper respiratory infection.23,31 Role of the Pharmacist Pharmacists should check for compliance, observe for effectiveness and adverse effects of therapy, and educate patients about what to expect from his or her drug(s). The pharmacist is in an ideal position to help patients understand how medical and nonmedical therapies work together. Many patients will also seek out treatment from alternative or nontraditional sources. In this regard also, the pharmacist is in the best position to inquire about all therapies and help patients integrate them, while helping to minimize unnecessary healthcare expenses. It is also important to stress that proper management of OA can only be accomplished after a correct diagnosis has been made. Many OA patients do not seek treatment from a physician because they assume that they are just “getting old” or “nothing can be done about arthritis anyway.” Therefore, an essential role of the pharmacist is to assure that the patient is under the care of a physician and is aware of his or her exact diagnosis.
Monitoring Therapy: The evaluation of pain can be done simply and reliably using a visual analog scale, in which the patient is asked to rate the severity of pain on a scale, such as 1 to 10. Other simple measures include the measurement of grip strength if the hands are involved, or 50-foot walking time if the hips or knees are affected. Arthritis-specific quality-of-life questionnaires provide a more sophisticated measure of disease impact. Monitoring of adverse effects depends on the drug therapy chosen.
Usually, direct patient questioning is the most effective means to obtain information.
Initially, patients may be asked whether they are having any problems with their
medication. Next, direct questions can be used to establish whether specific side effects
have been noticed. With both older and newer NSAIDs, symptoms of abdominal pain,
heartburn, nausea, and change in stool color are valuable for identifying GI complaints.
Patients can be asked about possible weight gain (from sodium retention), rash or
drowsiness. Conclusion Pain is the main symptom limiting function and quality of life for OA patients. Because OA patients are generally elderly and have comorbid conditions, it is essential to use the safest possible treatments. The American College of Rheumatology guidelines emphasize that acetaminophen is the drug of first choice in OA. However, many patients will require the substitution or addition of an NSAID. NSAIDs should be used as needed in the lowest dose possible, along with nonpharmacologic therapies and topical treatments. Although not proven, alternative agents such as glucosamine/chondroitin or hyaluronic acid may be an option for some patients. For patients at higher risk of GI ulcers, COX-2 selective inhibitors offer documented pain relief and convenient dosing, with the likelihood of fewer side effects than with the nonselective NSAIDs. References
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||