

Thesis Proposal
The Petroleum and Petrochemical College
Chulalongkorn University
Thesis title : Purification of i-C4 Obtained from Natural Gas
Thesis for : Master Degree in Petrochemical Technology
Name of student : Mr. Somchai Kwandee
Student I.D. no. : 41710290
Name of advisors : Dr. Vivan Thammongkol
Dr. Kitipat Siemanond
Academic year : 1998-1999
Date of preparation : March 29,1999
Student signature : .………………………………………………………………….……….
Approved by : .………………………………………………………………….……….

Purification of i-C4 Obtained from Natural Gas
Introduction
Natural gas from the gulf of Thailand is separated by gas separation plant to improve the quality of products such as methane, ethane, propane, LPG and NGL. Nowadays, Petroleum Authority of Thailand (PTT) has increased capacity by constructing gas separation unit up to 4 units, which can serve the demand of petrochemical industries, domestic energy consumption and also export to China, Vietnam, Singapore and Indochina countries. According to total productivity from 4 units, LPG is produced with the highest capacity of approximately 900,000 tons per year and the next ones are ethane and propane with the capacity of approximately 500,000 tons per year[1]. With high LPG productivity, PTT has concentrated in improving the value of LPG via separating out isobutane to get the new product such as the refrigerant, which is more valuable and can serve the customer's demand.
In isobutane separation, it is necessary to design a new process. A simple and economical method by process study is the computer simulation, which can exhibit optimum operating condition, capital cost and economic capacity. This is a track to make the decision about the possibility of investment. The PRO/II is selected to use as the simulation software. This software has the high accuracy and is widespread in simulation techniques of many leading companies.
This study is classified into 2 parts: part I is a simulation of distillation technique especially a deisobutanizer column and part II is a simulation of the other separation techniques such as absorption and membrane separation. In this study, the input data using the real data from gas separation plant and various parameters are varied until the purity of isobutane reaches approximately 99.7 % purity and the optimum point of operating condition. The simulation results will help the design engineer to design the optimum process before doing the real scale design.
Objective
To obtain 99.7% purity of isobutane for refrigerant.
Scope of Research Work
Do simulation or search for modified purification process or separation technique in order to obtain 99.7% purity of isobutane.
Related Research Works
O’Connell et al.[2] was patented an n-butane/isobutane splitter which is operated by compressing the isobutane overhead to increase its condensing temperature, using the compressed overhead to heat bottoms in a reboiler, which is operated to condense the overhead and cooling the condensed overhead to a temperature no lower than the temperature on the top tray of the splitter and no higher than 20oF above the temperature on the top tray, whereby the throughput of the splitter is increased by 10 to 20%. A feed suitable for this procedure would contain 5 to 95 % by moles of isobutane, 5 to 95 % by moles of n-butane, 0 to 20 % by moles of butenes, 0 to 5 % by moles of C3’s and lighter, and 0 to 5 % by moles of C5’s and heavier. This splitter can obtain isobutane purity in excess of 95 % by moles.
Schorre et al.[3] was patented a C4 separation process, which is directed to the conservation of energy by the use of an open heat pump to supply and portion of the heat to an n-butane/isobutane splitter as the compression fluid in the heat pump. A problem, which would be expected to prevent this system, is the unusual characteristic of n-butane (also isobutane) that on compression a liquid-vapor phase results, which is damaging to the compressor. This is overcome by passing the vaporized n-butane prior to compression, through an apparatus that remove any entrained liquids and heats the vapors to a temperature sufficient to prevent the formation of the liquid phase under compression. A feed suitable for this process would contain 5 to 95 % by moles of isobutane, 5 to 95 % by moles of n-butane, 0 to 20 % by moles of butenes, 0 to 20 % by moles of butadiene, 0 to 5 % by moles of C3 and lighter hydrocarbons, and 0 to 5 % by moles of C5 and heavier hydrocarbons. This process can obtain purity of isobutane reaches approximately 96.13 % by moles.
Vora et al.[4] was patented a process for a separation of isobutane from an alkylation reaction zone hydrocarbon effluent stream comprising isobutane, n-butane, propane and alkylate. The hydrocarbon effluent stream is charged to an isostripper column. An isobutane vapor stream from the column is condensed in indirect heat exchange with the lower liquid stream from the isostripper column comprising n-butane. The lower liquid stream is flashed in indirect heat exchange with the isobutane vapor stream at conditions to provide a vapor phase, the isobutane vapor phase being compressed and recycled to the isostripper column at a temperature to promote vapor formation.
Manley [5] was patented a process which enhance the use of partial effects and component distribution regulating NGL fractionation, especially relating to the separation of propane, isobutane, normal butane and gasoline components. In a partial effect de-isobutanizer has a two effect deisobutanizer, with one effect only making a partial separation in order to balance the energy load of the columns fractionation plant with relatively little additional investment cost. A feed of this unit is a natural gas liquid (NGL) from which methane, ethane and propane are reduced to levels then permitting recovery of isobutane and butane at a desire purity.
Schneider et al.[6] studied the analysis of alky unit deisobutanizer (DIB) expose design and operating conditions. The DIB feed is fractionated into three products: isobutane in the column overhead is recycled to the alkylation plant reaction section, alkylate in the column bottom is sent to gasoline blending and normal butane is removed in a vapor side draw. The authors gathered and analyzed the unit operating data by developing a process simulation to match the operating data. The data revealed two major areas of concern: the bottom-reboiler inlet temperature was 10 oF greater than the alkylate product temperature and the 250 psig steam supply to the bottom reboiler had about 70oF of superheat. They found that with half of the normal reboiler feed did not enter the exchanger. This bypass of the reboiler inlet had several detrimental effects on reboiler performance. It reduced the separation efficiency of the reboiler to much less than one theoretical stage. The calculated heat transfer coefficients for the bottom and side reboilers were about 70% of design. This cause from the fouling that can occur in an alkylation unit as a result of poor reaction-zone conditions. The authors suggest the company to improve the process by 4 improvements: installing a baffle and modified seal pans in the column to ensure that the bottom tray liquid was preferentially fed to the bottom reboiler, cleaning both reboilers to eliminate the fouling, putting a steam-header desuperheater into service and modifying the internals to provide the preferential feed to the side reboiler. After implementing this improvements, unit performance improved and profitability was increased.
References
[1] Petroleum Authority of Thailand (PTT) website, http://www.ptt.or.th.
[2] Harry E. O’Connell, James O. Nye, 1988, N-butane/isobutane fractionation, Enterprise Products Company, US Pat. Appl. 940,770.
[3] Kenneth R. Schorre, James O. Nye, Dennie W. Dixon, Carl Nepute, 1982, C4 separation porcess, Tenneco Oil Company and Nye Engineering Incorporated, US Pat. Appl. 186,515.
[4] Bipin V. Vora, Anthony G. Vlekers, 1981, Method of operating an isostripper column, UOP Inc., US Pat. Appl. 181,032.
[5] David B. Manley, 1998, Multiple effect and distributive separation of isobutane and normal butane, US Pat. Appl. 628,454.
[6] Donald F. Schneider, Joseph Musumeci, 1996, Analysis of alky unit DIB expose design operating consideration, Oil& Gas Journal, Vol. 94, No. 40, pp. 41-44.
Methodology
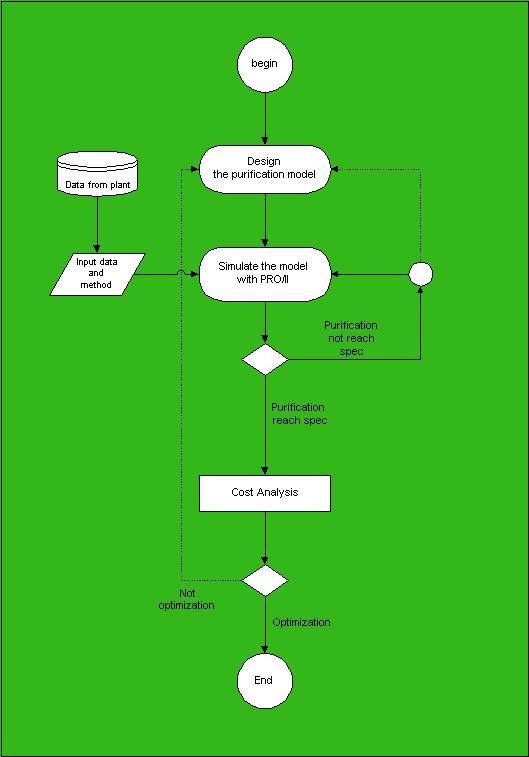
Flow diagram of the simulation
Data for simulation
Experimental plans
Schedule of Research Activities
Month |
Feb |
Mar |
Apr |
May |
Jun |
Jul |
Aug |
Sep |
Oct |
Nov |
Dec |
Jan |
Feb |
1 |
|||||||||||||
2 |
|||||||||||||
3 |
|||||||||||||
4 |
|||||||||||||
5 |
|||||||||||||
6 |
|||||||||||||
7 |
Activities
Budget of Thesis
price/unit unit price
Instrument
1. Personal computer/PC - 1 available
2. PRO/II software package - 1 available