

Thesis Proposal
The Petroleum and Petrochemical College
Chulalongkorn University
Thesis title : Adsorption of surfactants on ink related to paper recycling
Thesis for Master Degree in Petrochemical Technology
Name of student : Mr. Thitipong Kornprapakul
Student I.D. no : 4171035063
Name of advisor : Prof. J. F. Scamehorn
Name of co-advisor : Assoc. Prof. Kunchana Bunyakiat
: Dr. Kitipat Siemanond
Acadamic year : 1998
Date of preparation : 15 March 1999
Student signature :
Approved by :



Introduction
The use of recycled wastepaper can have numerous environmental and operating benefits compared to the various virgin pulp production methods. It has been estimated that for every ton of paper, which is made from 100% recycled wastepaper, 24 trees is saved. One ton of pulp made from deinked and bleached wastepaper requires 60% less energy to manufacture than a ton of bleached virgin kraft pulp (Thompson, 1992). Other benefits of wastepaper recycling are correlated with reduced capital and operating costs, reduced cost of purchase of raw material, reduced cost of energy and chemical used, extension of municipal landfill life, and the flexibility of the mills to sell to customers who prefer or need recycled paper (Thompson, 1992). In the near future, it is expected that a significant amount of wastepaper recovered from municipal solid wastes will be used for this purpose, and the printed wastepaper will increase in value as a raw material for better grade of paper, if the deinking technology is sufficiently developed.
The efficient ink removal is necessary to recycle used paper into high value products. Paper recycling is a simple process, only two steps are necessary. First, the secondary fiber source must be shredded into contaminants and recoverable fibers. Next, the contaminants must be separated from the fibers. However, the practice of recycling involves many complicated steps (Estes, 1995). Varying types and concentrations of contaminants can hamper the recycling process. Ink removal requires chemical, mechanical and thermal energy. For example, thermal energy (pulping temperature) affects the rate and completion of surfactant promoted the ink detachment from cellulose fibers. Mechanical energy influences the amount of surfactant promoted foaming in various stages of the deinking process.
Two principal deinking systems have been developed by paper industry: wash deinking and flotation deinking. For wash deinking the ink particles are separated from pulp fiber by formation of dispersion of ink and removing the dispersed ink by washing process. For flotation deinking, the ink particles are detached from pulp fiber by froth flotation, but small ink particles are not removed from the pulp, therefore the yield of deinked pulp is high but the physical strength of deinked pulp is poor. Many deinking mills try to incorporate the technology of flotation deinking into two-stage (washing and flotation) system to increase the efficiency and higher-grade end products.
In flotation deinking, generally fatty acid (such as oleic acid) is used as surfactant and also is combined with calcium, which acts as an activator, but the exact mechanism of the interaction of calcium with surfactant, and with the ink is not well understood. This thesis will focus on the adsorptive mechanism of surfactant and co-adsorption of calcium on the ink.
Objectives
Scope of research work
This thesis research is a laboratory experiment. The study is to investigate the adsorption characteristics of surfactants and co-adsorption of calcium on the ink, and effect of variables. In this study case, surfactant type, pH and calcium concentration will be investigated. The experimental data will be used to propose the adsorption isotherms of surfactants on ink.
Sodium octanoate, which represents medium chain length carboxylates, and sodium dodecyl sulfate will be used as surfactants, which adsorb on the model ink (carbon black) or similar model ink, instead of real ink that consists of varying pigments an binders and will use calcium chloride as co-adsorption. The simplified system will be selected to elucidate the mechanism without interference from extraneous variables.
The experiments will work on the test tubes. In all the adsorption experimentation, the concentration of the calcium and surfactant will be remained below the Ksp (solubility product equilibrium constant) value that no precipitation of surfactant occurred. The surfactant concentration will be remained below the CMC (critical micelle concentration) for all experiments.
Literature survey
Larsson et al. (1984) studied the zeta potential and flotation efficiency as a function of the addition of calcium ion concentration to model ink dispersions in the presence of sodium stearate. The investigators observed that absolute value of negative zeta potential of ink particles decreased with increasing calcium concentration while the flotation efficiency increased. Microscopic analysis of the ink particles revealed that precipitated calcium distearate adhered to the surface of the ink. They concluded that precipitation of calcium dicarboxylate created a micro-encapsulation of ink particles causing aggregation, hydrophobicity and subsequent flotation.
Hornfeck (1987) advocated the existence of an electrostatic calcium bridge that was formed between the moderately negative surface of the ink particle and anionic head group of carboxylate surfactant. The hydrophobic tails of surfactants were then free to interact with the hydrophobic portions of other such bridged surfactants, thus causing ink particle aggregation due to the hydrophobic bonding. Particles and aggregate attachment to air was explained by adsorption the hydrophobic tails of the surfactant molecules at the air-water interface of rising air pocket.
G. M. Dorris and N. Ngugen (1995) studied flotation of model inks (flexographic inks) without fibers. Flexographic inks, which were composed of a pigment, usually carbon black that dispersed in water with the use of water-soluble carboxylic derivatives, which also performed as binder. The results showed that, in basic condition, the carboxylic groups led to destabilization of ink particles and to higher flotation rates. The floatability of flexographic inks at alkaline condition improved with the addition with calcium ions and much more so with the addition of both sodium oleate and calcium ions, under turbulent agitation. The results suggested that conventional calcium soaps of fatty acids were effective flotation collectors for flexographic inks.
A. P. Oliveira and M. L. Torem (1995) studied the influence of some metallic cations on deinking flotation. This study presented some investigations on the adsorptive mechanisms of some anionic collectors, such as sodium stearate, oleic acid, and sodium dodecyl sulfate on ink particles, in the absence/ presence of some metallic cations, calcium, magnesium, and aluminium ions. The study revealed that the presence of metallic cations modified zeta potential curves of ink particles. The activating effect of metallic cations was probably associated with calcium ion species for calcium and the hydroxy complexes and positively charged metallic hydroxide for magnesium and aluminium. The morphology of ink particles depended upon the solution chemistry. In the absence of metallic cations, ink particles formed aggregates with rough surfaces (size range 40–200 m m). In the presence of metallic cations, aggregates had smoother surfaces (15–35 m m).
Achille E.Riviello, Jr. (1997) studied ink removal from secondary fiber by flotation deinking. The study focused on two medium chain-length fatty acids (sodium octanoate and sodium dodecyloctanoate) and a comparable chain-length sulfate (sodium dodecyl sulfate). The system behavior was observed below Ksp (solubility product equilibrium constant) of surfactant/ calcium complex. By investigating the adsorption isotherms of surfactants on both model inks and fibers, the zeta potential, the aggregation characteristics of model ink, and the flotation of the inks and fibers. The fundamental mechanisms were found to be adsorptive rather than precipitative. This understanding could translate into more efficient surfactant formulation for the flotation deinking process.
R. N. Rao and P. Stenius (1998) studied the mechanisms of ink and the influence of several nonionic surfactants on release of ink from cellophane, a polyamide sheet and photocopy paper, were used as model substrates. A simple model of the structure of dry ink, which was assumed to consist of a primary layer (directly bound to the substrate), a secondary layer, and tertiary layer, was used to elucidate how ink detachment takes place. They found that the addition of surfactant was crucial because it softened the ink and affected the ink/fiber interactions. For complete detachment of ink generally required mechanical force, however mechanical action alone could not successfully remove ink in direct contact with the fibers. Moreover, they indicated the structural difference in surfactants might affect kinetics rather than actual detachment mechanism.
Niall Robertson et al (1998) studied washing the pulp fibers from flotation froth for increasing yields in flotation deinking. In this process, air is sparged into bottom of a column, which is filled with sodium dodecyl sulfate (SDS), and a wash water stream sprayed on the top of the column. The concentration of fibers that broke through into the liquid area below froth was measured. The results revealed that it was possible the pulp yield by washing the froth. The froth washing could prevent fiber loss during flotation deinking. The fiber transport was by a slip-stick behavior, which was impelled by slug of water, and the longer fibers had greater tendency to be kept in the foam.
J. Y. Zhu et al (1998) studied flotation deinking of toner-printed papers by using frother spray. Instead of adding frother into the pulp slurry directly before flotation in the conventional process, a pressurized atomizer was used to spray the frother solution from the top of the column. The pulp was made from xerographically copied bond paper and Triton-100 was used as frother. The results showed that the frother spray approach could effectively establish a stable froth for good ink removal. It could reduce fiber loss of 50%, water loss of 75% and frother consumption of 95% without sacrificing in deinking efficiency. It could be the effective method of improving the effectiveness of dispersant, collector, and frother in flotation deinking.
References
Methodology
Experimental Set-up
The carbon black (type 4 0 0 R) will be used as model ink. Due to a relatively high concentration of ionic salt present, it will be necessary to wash the carbon to remove salt. Carbon black will be mixed with distilled or deionized water in the ratio 1:4, agitated thoroughly, centrifuged, and water decanted off. This procedure will be repeated 3 times, which will be sufficient to reduce the calcium concentration below 0.1 ppm that measured by atomic absorption spectroscopy technique.
The surfactants employed for the investigation will be sodium octanoate and sodium dodecyl sulfate. Prior to use surfactants, they will be recrystallized out of ethanol and water. From Daryl (1 9 8 5 ), the recrystallization will be performed by placing the solid surfactant in the reagent grade ethanol and adding just enough water to completely dissolved all of the surfactant at 45 oC. The solution will be filtered to remove any inert materials, and then placed in the refrigerator for approximately 2 days. At the end of two days, the recrystallized surfactant will be removed from the refrigerator and filtered in a fritted glass funnel. The crystals will be dried under the vacuum to remove the excess water and ethanol. This procedure will be standard practice in the laboratory.
Calcium chloride will be oven dried for 12 hours at 90 oC just prior to manufacturing the stock solution due to the chemical hygroscopic nature.
Sodium hydroxide and hydrochloric acid will be used as pH adjustment.
Water used for experimentation will be distilled or deionized.
2. Experimental procedure
Adsorption isotherm experiments
The adsorption isotherms will be obtained at 30 oC using the solution depleting method. The amount of adsorbed surfactant and/or adsorbed calcium ion will be calculated by performing a mass balance between initial and final surfactant and/or calcium concentration. Volume of suspension and quantity of ink will be remained constant and concentrations of surfactant and calcium ion will be varied. The supernatant portion above the ink will be clarified by using a centrifuge, and will be filtered to remove all suspended solids. The filtrate will be kept for further analysis.
Controlled parameters
For adsorption isotherm experiment, the volume of suspension, quantity of fiber and ink, and operational temperature will be kept constant.
Variable parameters
This research will study the effect of surfactant type, pH, and calcium concentration on the performance of adsorption process.
Method of chemical analysis and measurement
For investigation calcium ion concentration, atomic absorption spectroscopy technique will be required. High performance liquid chromatography (HPLC) will be used to determine the sodium dodecyl sulfate (SDS) concentration. But in the sodium octanoate concentration, total organic carbon analyzer (TOC) will be required.
Data calculation and analysis
![]()
For this thesis, Equation (1) will be used to calculate the amount of surfactant adsorbed onto the surface of ink.
where
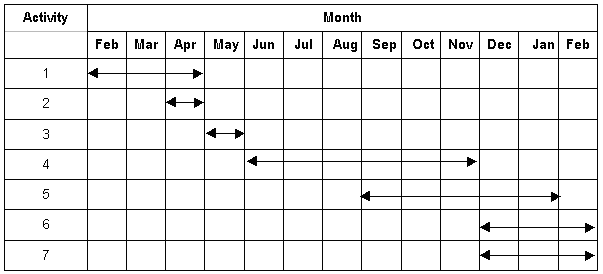
Schedule of Research Activities and timetable
Sequence of research activities
1.Literature survey
2. Checking for the exist equipment and ordering for additional chemical and apparatus.
3. Experimental set-up.
4. Running the experiments.
5. Data analysis.
6. Making the project conclusion.
7. Writing the complete report and paper.
Time table
Year : 1999
Budget
Cost (Baht)
Chemicals
Carbon black ---------------------------------------------------------------------------10,000
Sodium dodecyl sulfate ---------------------------------------------------------------8,500
Sodium octanoate --------------------------------------------------------------------10,000
Ethanol -------------------------------------------------------------------------------------4,500
NaOH ---------------------------------------------------------------------------------------2,500
HCl -------------------------------------------------------------------------------------------2,500
DI water -------------------------------------------------------------------------------------3,500
Total ------------------------------------------------------------41,500