CHAPTER IV
RESULTS AND DISCUSSION
4.1 Surfactant adsorption isotherms
There are several factors strongly influencing surfactant adsorption at the solid-liquid interface such as nature of structural groups on the surface, molecular structure of surfactant and the environment of aqueous solution. For this thesis, pH of system and electrolyte content (calcium concentration) were selected to elucidate the adsorption behavior of surfactant on carbon black.
4.1.1 Effect of pH of suspension
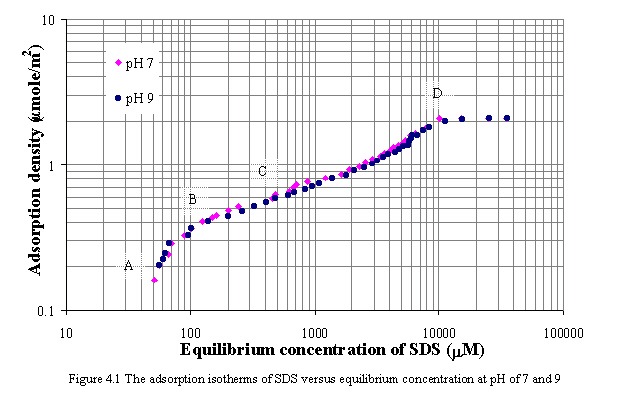
In this section, pH of 7 and 9 were selected for moderate basic pH condition to imitate conditions of traditional deinking operations. Figure 4.1 illustrates the adsorption of sodium dodecyl sulfate (SDS) versus equilibrium concentration at pH of 7 and 9 in the absence of calcium. Comparing the adsorption isotherms, the adsorption at pH of 7 is slightly greater than the adsorption at pH of 9 in all regions. As the pH of the aqueous phase is reduced, the solid surface becomes more positive or less negative due to the adsorption of protons onto charged sites. This consequence leads to increase in the adsorption of anionic surfactants. For carbon black in water, the point of zero charge (PZC) was determined to be approximately 2.3 indicating that the carbon black has a net negative charge at pH levels above 2.3 (Riviello et al., 1997).

Results of adsorption isotherms on carbon black at both pH of 7 and 9, plotted on log-log scale, are different from typical adsorption isotherms, which are observable the four distinct regions. At small equilibrium SDS concentration (from point A to B shown in Figure 4.1), the isotherm shape is similar Langmuir-type isotherm. The slope of isotherm increases and fairly constant at point B. It is expected that the monomeric surfactants adsorb on the surface without significant association or aggregation of adsorbed surfactants. The horizontal or laying-down configuration of adsorbed surfactants is possible to occur, the interaction of adsorbed surfactants is a result of hydrophobic chain/surface interaction. The adsorption of surfactants in this orientation may remain until complete monolayer coverage. Beyond this stage, the slope of isotherm is steep again as increasing surfactant concentrations. The marked inflection point occur at C. The adsorption of surfactants is more close packing, and the interaction of adsorbed surfactants may be hydrophobic chain/chain interaction. However, from log-log adsorption isotherm at pH of 7 and 9, the slope of such area (from point C to D) is not greater than unity. It may be indicated that the adsorption of SDS on carbon black is mainly unassociative or at least not strongly associative. The plateau or maximum adsorption of SDS at pH of 7 and 9 is approximately 2.06 mmole/m2 and occurs near CMC of SDS, 8300 mM (Mukerjee et al., 1970).
The approximate adsorption corresponding to saturation of surface by either a monolayer or a bilayer can be determined from the area of single adsorbed surfactant molecule. Rosen (1978) reported that an area of 50 Ao2 for sulfate molecule adsorbed in close-packed perpendicular orientation on surfaces such as carbon black. Calculating this value, the close-packed monolayer coverage would require 3.2 mmole/m2. The plateau adsorption from the experiment corresponds to 65% of monolayer coverage for SDS.
4.1.2 Effect of calcium concentration
In the adsorption and electrophoretic experiments, SDS and calcium concentrations were controlled without precipitation of calcium didodecyl sulfate complexes. On the phase boundary, the precipitate phase is in equilibrium with dissolved surfactant and calcium. For systems consisting of a divalent cation such as Ca2+ and a monovalent anionic surfactant, the concentration-based solubility product constant (Ksp) is defined as follows;
Ksp = [Ca2+][DS-] 2
where the bracketed values represent the concentration of species in the solution at equilibrium with the calcium didodecyl sulfate precipitate. The concentration-based Ksp of calcium didodecyl sulfate at 30oC was 6.0x10-10 M3 (Riviello et al., 1997) and the activity-based Ksp was 5.02x10-10 M3 (Stellner et al., 1989). Figure 4.2 and 4.3 depict the adsorption of SDS on carbon black at constant pH of 7 and 9 as varying calcium concentrations. The initial calcium concentrations were varied from 100 to 1000 mM. In all experiments, the concentrations of calcium and SDS at equilibrium remain below the Ksp value. As increasing calcium concentration levels, the adsorption of SDS on carbon surfaces increases. Because an increase in ionic salt lead to a decrease in the repulsive forces between the head groups of surfactants. Moreover, decreasing the electrical repulsion between the similarly charged adsorbed ions permits closer packing. From the experiments, the plateau adsorptions of SDS at initial calcium concentration of 100, 700, and 1000 mM are 2.10, 2.11 and 2.18 mmole/m2, respectively for pH of 7 and 2.08, 2.09, and 2.13 mmole/m2, respectively for pH of 9.
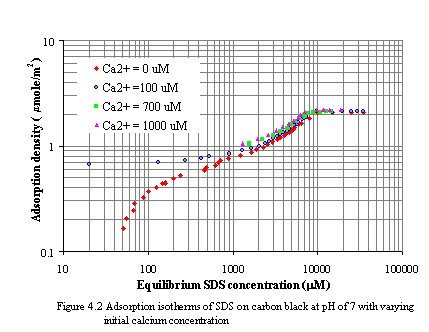
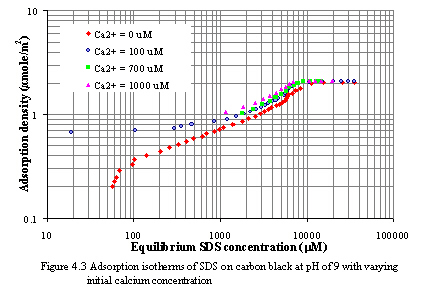
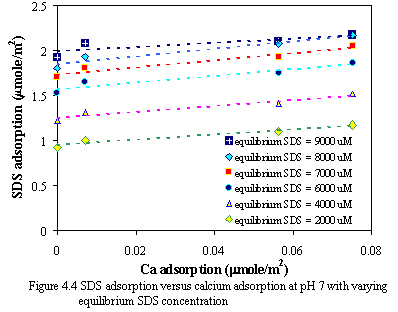
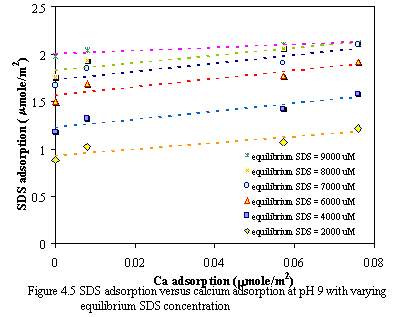
Figure 4.4 and 4.5 demonstrate the relationship of calcium adsorption and SDS adsorption with varying equilibrium SDS concentration at pH of 7 and 9. The results show that at constant equilibrium SDS concentration when the adsorption of calcium increases, the adsorption of SDS also increases in the same way, when the adsorption of SDS increases, the adsorption of calcium also increases. This may indicate the cooperative adsorption with ionic surfactants in the presence of oppositely charged ions. The electrostatic repulsion is diminished among the head groups of surfactants by association of the counterion, and the link is not precipitation process.
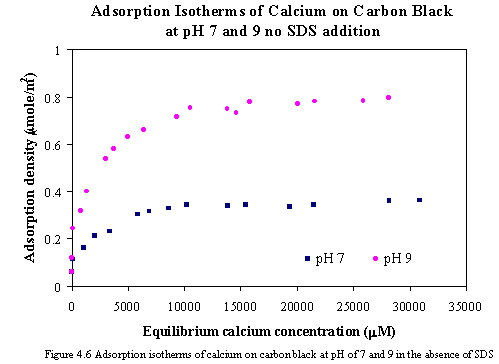
The adsorption of calcium on the carbon black in the absence of SDS at pH of 7 and 9 is shown in Figure 4.6. The result of calcium adsorption isotherms is Langmurian shape, indicating that the adsorption of calcium is monolayer coverage. The adsorption of calcium ions is attracted by negatively charged sites on the carbon surfaces resulting from surface oxidation. The ionic radius of calcium is 0.099 nm (Russell, 1992). The close-packed monolayer coverage of adsorbed calcium from calculation is 167.8 mmole/m2. The plateau adsorptions of calcium ions on carbon surface at pH of 7 and 9 were approximately 0.36 and 0.79 mmole/m2 corresponding to 0.21% and 0.47% of close-packed monolayer coverage respectively. Generally, bare cations are smaller than anions, and they bond tenaciously to hydrating water molecules (Oldman K. B. et al., 1994). The interaction between adsorbed calcium ions is not able to occur since aqueous molecules surround adsorbed ions and the lateral interactions are repulsive, not attractive. Consequently, it can be concluded that the adsorption of calcium on carbon surfaces is purely electrostatic and non-associative.
4.1.3 Electrophoretic determination
Zeta potential is an approximation of surface potential. It is the electrical potential at the shear plane between Stern layer and Diffuse layer. It is an important feature because zeta potential can be measured in a fairly simple manner, while the surface potential cannot. Zeta potential is an effective tool for coagulation control because of changes in the repulsive force between colloids.
Figure 4.7 illustrates the zeta potential of carbon black as a function of equilibrium SDS concentration without calcium addition at pH of 7 and 9. The results indicate that SDS has the effect on the measured zeta potential. While the equilibrium SDS concentration increases, the absolute zeta potential of carbon also increases. Since the adsorbed SDS on carbon exposes the negatively charged group to the solution, and the hydrophobic tail group interacts with the surface, therefore the absolute zeta potential of carbon increases as increasing SDS concentration. At constant equilibrium SDS concentration, the absolute zeta potential of carbon at pH of 9 is slightly greater than one at pH of 7. Because the effect of pH between 7 and 9 is not significant to the SDS adsorption on carbon surface, the net negative charge on carbon surface at pH of 9 may remain higher than one at pH of 7.
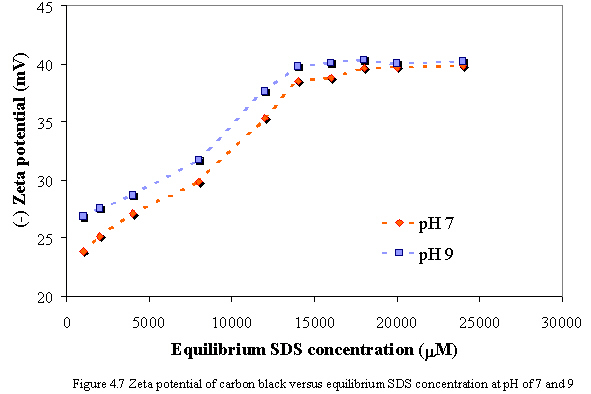
Figure 4.8 and 4.9 depict the zeta potential of carbon versus equilibrium SDS concentration as varying calcium concentration at pH of 7 and 9. With constant equilibrium SDS concentration, the absolute zeta potential of carbon decreases as increasing calcium levels. It is probably because calcium ions neutralize the negatively charged sites on carbon surfaces and hydrophilic groups of SDS, consequently, the net negative charges on carbon adsorbents decrease.
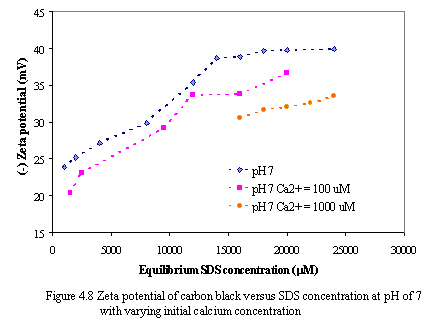
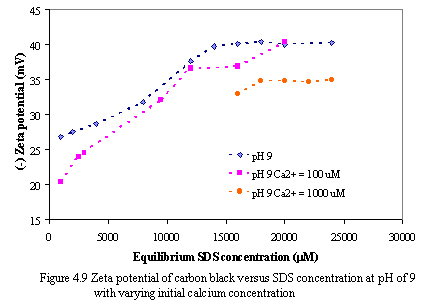
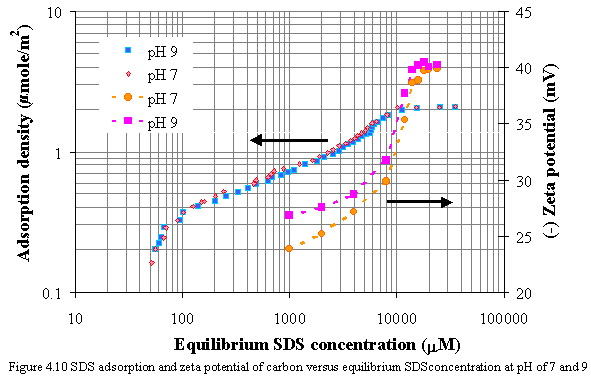
Figure 4.10 shows the relationships of adsorption isotherms and zeta potential versus equilibrium SDS concentration at pH of 7 and 9 in the absence of calcium. At low SDS adsorption, the negative zeta potential increased slightly from -23.8 to -27.1 mV at pH of 7 and from -26.8 to -28.7 mV at pH of 9. After that the negative zeta potential decreased significantly from -27.1 to -38.6 mV at pH of 7 and from -28.7 to -39.8 mV at pH of 9 as increasing SDS adsorption more. Finally, the zeta potential is fairly constant as SDS concentration slightly beyond the CMC of SDS.
4.2 Calcium adsorption isotherm
The purpose of this experimental part is to study the role of small concentrations of SDS to calcium adsorption on carbon black. Many research works indicate that electrolytes or counterions show dramatically on the adsorption of surfactants. Addition of neutral electrolyte such as NaCl or KBr causes a decrease in the adsorption of the ionic surfactants onto the oppositely charged adsorbents and an increase in their adsorption onto the similarly charged adsorbents. Conversely, the addition of surfactants such as anionic surfactants should obtain whether positive or negative effect on the adsorption of counterions.
4.2.1 Effect of surfactant concentration
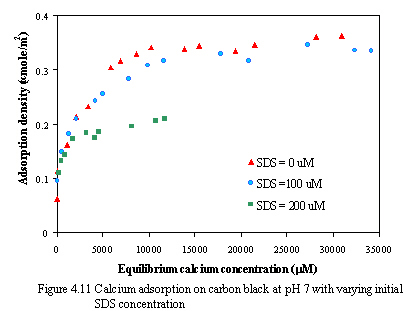
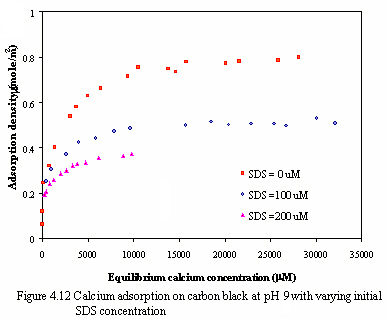
Figure 4.11 and 4.12 illustrate the effect of SDS concentration on the adsorption of calcium at pH of 7 and 9. The results of calcium adsorption isotherms at varying SDS concentration are Langmurian curves. And the adsorption of calcium ion decreases as increasing small amount of SDS concentration. Because the monomeric SDS probably adsorbs onto carbon surface in the horizontal or laying-down configuration, some parts of hydrophobic tail group may locate at negatively charged sites which may consequently be the barriers for calcium ions to adsorb the remaining negatively charged sites as calcium adsorption decreases. Another possible reason to explain is that the amount of SDS added is very small, when the surfactant adsorbs on the surface without the interaction between individual adsorbed surfactants, the cooperative adsorption with ionic surfactants in the presence of ionic salt rarely occur. From the result, the adsorption of calcium on the carbon in the presence of small amount of SDS is less than the adsorption of calcium without SDS addition.
4.2.2 Electrophoretic determination
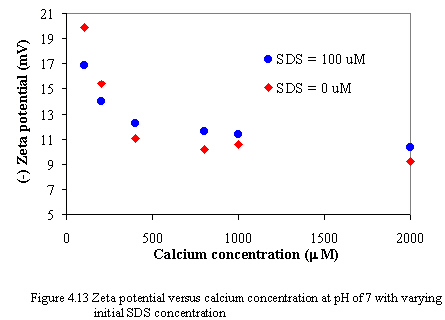
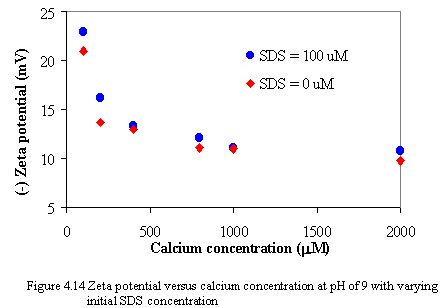
Figure 4.13 to 4.14 illustrate the zeta potential of carbon black versus equilibrium calcium concentrations as varying SDS concentration at pH of 7 and 9. When calcium is added to the system, the magnitude of zeta potential decreases dramatically because the adsorption of calcium onto oppositely charged sites of carbon surfaces results in compression of electrical double layer and lowering of electrical potential of carbon that is the zeta potential of carbon also decreases. However, at the constant calcium concentration, the change in the magnitude of zeta potential is small as increasing amount of SDS. It is possibly because the amount of SDS added is small that it may be no effect on changing the magnitude of the zeta potential.